- PRODUCTS產品介紹
- Cell Culture Cell Culture
- Animal Cell Culture Animal Cell Culture
- Insect Cell Culture Insect Cell Culture
- Stem Cell Culture Stem Cell Culture
- Immune Cell Culture Immune Cell Culture
- Antibiotic/Cytokine/Growth Factor Antibiotic/Cytokine/Growth Factor
- Cell Preparation Cell Preparation
- Cell Storage/Transportion Cell Storage/Transportion
- Cell Culture Supplements Cell Culture Supplements
- Cell Dissociation & Buffer Solutions Cell Dissociation & Buffer Solutions
- Extracellular Matrix Extracellular Matrix
- Cell Isolation Cell Isolation
- Human Platelet Lysate Human Platelet Lysate
- Animal Cell Culture
- Cell Therapy Cell Therapy
- MSC
- NK
- CIK
- T Cell
- DC
- iPS
- Stem Cell-CD34 Stem Cell-CD34
- Adipose Stem Cell Adipose Stem Cell
- Water-Free Thawing System Water-Free Thawing System
- Blood biopsy preparation Blood biopsy preparation
- Quality Control Quality Control
- GMP Grade Cytokine GMP Grade Cytokine
- Common Chemicals & Buffers & Lab Tools Common Chemicals & Buffers & Lab Tools
- Life Science Life Science
- Exosome Research Exosome Research
- Spectradyne-Microfluidic Nanoparticle Analysis
- Corning-VideoDrop
- Immunostep- Lyophilized Exosome Standards
- Immunostep-ExoStep Platform
- Immunostep-Exosome Isolation Columns (SEC)
- GeneCopoeia Lentifect™ Exosome Labeling Lentiviral
- GeneCopoeia miProfile™ Exosome miRNA qPCR arrays
- Exosome Memebrane / Protein Dye
- DNA/RNA Research
- miRNA/shRNA/siRNA Research
- Protein Research
- In Vivo Assay In Vivo Assay
- Cloning & Clone Collection
- Transfection & Transduction
- IHC
- Cell-Based Assay
- Mitochondria Research Mitochondria Research
- Antibody/Antigen
- ELISA ELISA
- Antibody Labeling Antibody Labeling
- mRNA/Oligos/Nucleotide
- Microbial Research Microbial Research
- Ultra high content imaging
- Tumor Research Tumor Research
- Microarray Microarray
- Exosome Research
- Pharma Manufacturing & QC Pharma Manufacturing & QC
- Impurity Detection
- Micoplasma Detection Micoplasma Detection
- Endotoxin Remove Endotoxin Remove
- Visual Inspection Visual Inspection
- Mycobacteria Detection Mycobacteria Detection
- Nanoparticle Analyzer Nanoparticle Analyzer
- Cell Culture
- NEWS最新消息
- PROMOTIONS促銷活動
- SUPPLIER代理品牌
- AAjinomoto-iPSAbcamAbebioAbbexaAbcoreAlpha-TecAkadeum
- BBiCell ScientificBIO-HELIXBiolineBioLife SolutionsBio X Cell
- CCorningCYGNUSCompass BiomedicalCUSABIOCytori Therapeutics IncCANDOR BioscienceCreative BioMart
- DDojindo
- EExpression SystemsEastCoast BioElixirgen Scientific
- FFast Forward DiscoveriesFortius BioFisher Scientific
- GGoldBioGenlantisGeneCopoeia
- HHyTest Ltd
- IImmunostepiRealIrvine ScientificInVitriaImmuno-Biological Laboratories
- KKohjin BioKingfisher Biotech
- LLIPOSOMA ─ Clodronate Liposomes
- MMatrixome & NippiMedicagoMeridian Life Science
- NNonacus
- QQIAGEN
- PProtein ArkProFoldin-ProteomicsProSpec
- SSHIMADZUSignaGenScyTek Laboratories IncSMOBIOSpectradyne
- TTriLink BiotechnologiesTymora Analytical
- WWorthingtonWaken X BioLife Solutions
- ABOUT關於我們
- CONTACT聯絡我們
- 產品介紹
- Cell Culture Cell Culture
- Animal Cell Culture Animal Cell Culture
- Insect Cell Culture Insect Cell Culture
- Stem Cell Culture Stem Cell Culture
- Immune Cell Culture Immune Cell Culture
- Antibiotic/Cytokine/Growth Factor Antibiotic/Cytokine/Growth Factor
- Cell Preparation Cell Preparation
- Cell Storage/Transportion Cell Storage/Transportion
- Cell Culture Supplements Cell Culture Supplements
- Cell Dissociation & Buffer Solutions Cell Dissociation & Buffer Solutions
- Extracellular Matrix Extracellular Matrix
- Cell Isolation Cell Isolation
- Human Platelet Lysate Human Platelet Lysate
- Animal Cell Culture
- Cell Therapy Cell Therapy
- MSC
- NK
- CIK
- T Cell
- DC
- iPS
- Stem Cell-CD34 Stem Cell-CD34
- Adipose Stem Cell Adipose Stem Cell
- Water-Free Thawing System Water-Free Thawing System
- Blood biopsy preparation Blood biopsy preparation
- Quality Control Quality Control
- GMP Grade Cytokine GMP Grade Cytokine
- Common Chemicals & Buffers & Lab Tools Common Chemicals & Buffers & Lab Tools
- Life Science Life Science
- Exosome Research Exosome Research
- Spectradyne-Microfluidic Nanoparticle Analysis
- Corning-VideoDrop
- Immunostep- Lyophilized Exosome Standards
- Immunostep-ExoStep Platform
- Immunostep-Exosome Isolation Columns (SEC)
- GeneCopoeia Lentifect™ Exosome Labeling Lentiviral
- GeneCopoeia miProfile™ Exosome miRNA qPCR arrays
- Exosome Memebrane / Protein Dye
- DNA/RNA Research
- miRNA/shRNA/siRNA Research
- Protein Research
- In Vivo Assay In Vivo Assay
- Cloning & Clone Collection
- Transfection & Transduction
- IHC
- Cell-Based Assay
- Mitochondria Research Mitochondria Research
- Antibody/Antigen
- ELISA ELISA
- Antibody Labeling Antibody Labeling
- mRNA/Oligos/Nucleotide
- Microbial Research Microbial Research
- Ultra high content imaging
- Tumor Research Tumor Research
- Microarray Microarray
- Exosome Research
- Pharma Manufacturing & QC Pharma Manufacturing & QC
- Impurity Detection
- Micoplasma Detection Micoplasma Detection
- Endotoxin Remove Endotoxin Remove
- Visual Inspection Visual Inspection
- Mycobacteria Detection Mycobacteria Detection
- Nanoparticle Analyzer Nanoparticle Analyzer
- Cell Culture
- 最新消息
- 促銷活動
- 代理品牌
- AAjinomoto-iPSAbcamAbebioAbbexaAbcoreAlpha-TecAkadeum
- BBiCell ScientificBIO-HELIXBiolineBioLife SolutionsBio X Cell
- CCorningCYGNUSCompass BiomedicalCUSABIOCytori Therapeutics IncCANDOR BioscienceCreative BioMart
- DDojindo
- EExpression SystemsEastCoast BioElixirgen Scientific
- FFast Forward DiscoveriesFortius BioFisher Scientific
- GGoldBioGenlantisGeneCopoeia
- HHyTest Ltd
- IImmunostepiRealIrvine ScientificInVitriaImmuno-Biological Laboratories
- KKohjin BioKingfisher Biotech
- LLIPOSOMA ─ Clodronate Liposomes
- MMatrixome & NippiMedicagoMeridian Life Science
- NNonacus
- QQIAGEN
- PProtein ArkProFoldin-ProteomicsProSpec
- SSHIMADZUSignaGenScyTek Laboratories IncSMOBIOSpectradyne
- TTriLink BiotechnologiesTymora Analytical
- WWorthingtonWaken X BioLife Solutions
- 關於我們
- 聯絡我們
News
【InVitria】OptiVERO Vero 細胞完整培養基套組
OptiVERO Vero 細胞完整培養基套組
疫苗製造商最完美選擇!
InVitria新推出OptiVERO,不需血清、不含任何血液衍生成分、不含動物衍生成分、不含植物水解物、化學成分確定的VERO細胞專用培養基。支持Vero細胞在T flask (2D culture) 與 microcarriers (3D culture) 的生長與擴增,並對於病毒生產進行了優化。
OptiVERO與市面上其他無血清VERO細胞培養基不同的地方在於,它甚至不含有未定義的二肽和三肽植物來源的水解產物,去除所有不確定的成分,將培養基中能引起的細胞擴增時的變異性降到最低,提高了疫苗研究和製造的一致性、準確性、安全性和可擴展性,從而加快生產時間。
研究成果於2018 World Vaccine Congress發表,此新型培養基表現出明顯優於市面上無血清培養基對VERO細胞的擴增能力和病毒生產,尤其是對於一些表達系統難以表達的病毒類型生產(如下圖)。
在流感病毒中,OptiVERO明顯表現出優於VP-SFM的生產能力,而登革熱和Zika病毒的產量與使用VP-SFM相當。
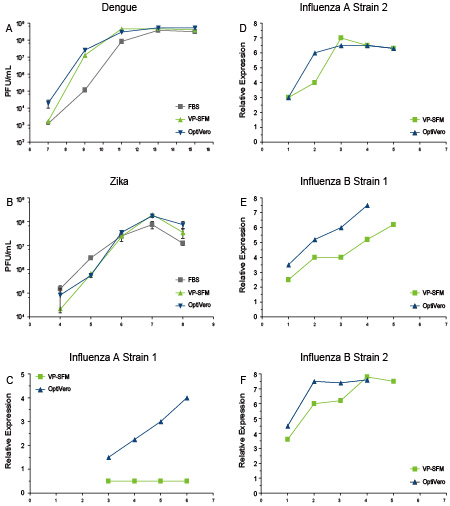
The OptiVERO media was subjected to productivity tests using a strain of Dengue and Zika flavivirus in WT VERO. For influenza, the genetically modified VERO subclone was utilized. WT VERO cells were grown for 3-4 days prior to infection. During the virus propagation phase, cultures were fed with a 45% glucose solution and the pH of the cultures were maintained at neutral using a 7.5% sodium bicarbonate solution. (A) FBS demonstrated a lag in the production of Dengue virus compared to VP-SFM and OptiVERO while (B) Zika virus did not demonstrate any significant differences between the media. Influenza virus demonstrated a higher degree of sensitivity to different media compared to the flavivirus Dengue and Zika. (C-D) VP-SFM failed to expand a strain of Influenza A and exhibited a significant delay in a second strain while OptiVERO was able to expand both of these virus strains very efficiently. (E-F) Influenza B strains 1 and 2 exhibited similar patterns in VP-SFM as this media induced a significant delay in the time to peak titer while OptiVERO was able to efficiently expand these virus types.
完整培養基套組包含基礎培養基、濃縮冷凍培養基和蛋白質補充劑。
經VERO細胞測試功能後出廠。
OptiVERO的優點包括:
- 降低細胞培養基的變異性
- 加速疫苗研究
- 提高疫苗製造的可擴展性和速度
- 降低製造時間和資源成本
現在可索取於2018 World Vaccine Congress發表的研究海報「Formulation of a blood-free and chemically defined virus production media for VERO cells」